Dive deep into the physics and chemistry explaining how Polaroids work. Understand the science behind instant film, from photons and silver halides to dye diffusion and image stabilization.
(Introduction) The allure of a Polaroid picture – emerging from the camera seemingly blank, then slowly resolving into a tangible memory right before your eyes – feels like magic. But behind this captivating process lies an intricate dance of physics and chemistry, a miniature, self-contained laboratory engineered by the genius Dr. Edwin Land and his team. While we often marvel at the instant result, understanding how Polaroids work from a physics perspective reveals a fascinating interplay of light, matter, and chemical reactions packed into a remarkably thin film pack. This article will explore the “A to Z” physics and chemistry principles that make instant photography possible.
Setting the Stage: The Instant Camera’s Role in Physics
Before the film’s internal processes begin, the instant camera performs crucial physics-based functions:
- Optics – Focusing Light: Like any camera, an instant camera uses a lens (or a series of lenses) to gather ambient light reflected off the subject. The fundamental physics of optics dictates how these lenses refract (bend) light rays to converge and form a focused, inverted image onto the film plane located inside the camera. The quality and complexity of the lens system directly impact image sharpness.
- Exposure Control – Quantifying Light: The camera must control the amount of light reaching the film. This involves:
- Aperture: An adjustable opening (like the iris of an eye) that controls the intensity of light passing through the lens per unit time. A wider aperture lets in more light (useful in dim conditions), while a smaller aperture lets in less (useful in bright conditions). This directly relates to the physics of light intensity and geometric optics.
- Shutter Speed: A mechanism (curtain or blades) that controls the duration for which light is allowed to hit the film. A faster shutter speed allows less total light, freezing motion, while a slower speed allows more light, potentially blurring motion. The total exposure is a product of light intensity (controlled by aperture) and time (controlled by shutter speed).
- Initiating Development: The unique function of an instant camera is ejecting the exposed film sheet and, in doing so, initiating the chemical development process. As the film exits, a pair of precise rollers applies uniform pressure, rupturing a pod of chemical reagent and spreading it evenly between specific layers within the film sheet. The physics of pressure and fluid dynamics are critical here to ensure consistent development.
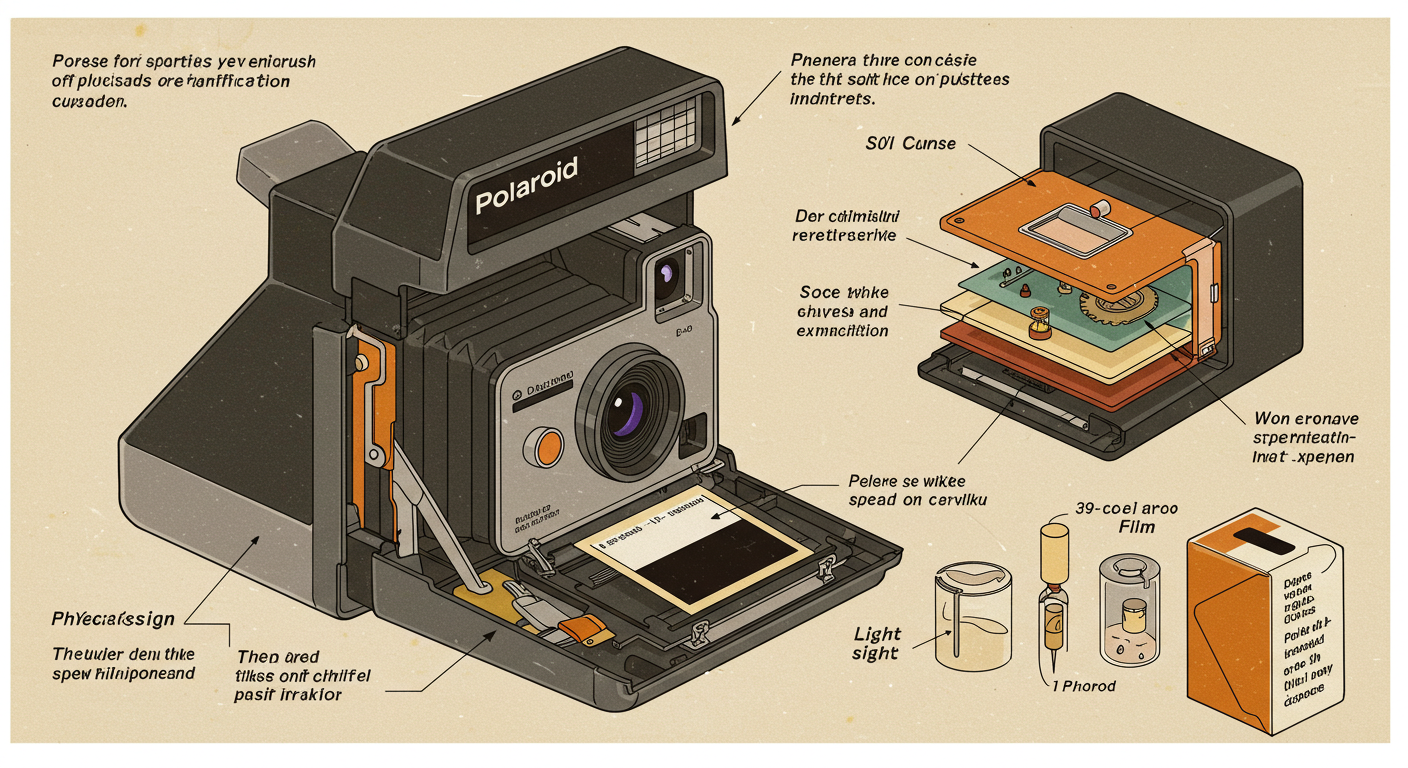
The Heart of the Matter: The Multi-Layered Instant Film Pack
The true marvel lies within the Polaroid film sheet itself. It’s not just a single piece of photographic paper; it’s a complex, multi-layered structure, essentially a miniature chemical plant. While specific formulations have evolved (from original Polaroid SX-70/600/Spectra to modern Polaroid I-Type/Go and competitor Instax films), the fundamental layered principle for integral color film (where the final image forms on the same sheet ejected) involves something like this (simplified, often viewed top-down as ejected):
- Clear Plastic Top Layer: Protects the image.
- Image-Receiving Layer: A crucial layer containing a ‘mordant’ – a chemical that traps and holds the diffusing dyes to form the final positive image.
- Timing Layer(s) & Acid Layer: These control the duration of the development process. The alkali from the reagent slowly penetrates the timing layer(s). Once it reaches the acid layer, a neutralization reaction occurs, stopping development. The physics of diffusion and reaction kinetics are key here.
- Reagent / Alkali Pod: (Initially separate, spread during ejection) Contains the highly alkaline processing chemicals, opacifiers (to protect the film from ambient light during development), and other agents.
- Light-Sensitive Layers (The Negative): This is where the image is initially captured. It consists of at least three emulsion layers, each sensitive to one primary color of light (blue, green, red), stacked with filter layers in between.
- Blue-Sensitive Layer: Contains silver halide crystals sensitive primarily to blue light. Associated with this layer are Yellow Dye Developer molecules.
- Yellow Filter Layer: Blocks blue light from reaching the lower layers. It becomes transparent during development.
- Green-Sensitive Layer: Contains silver halide crystals sensitive primarily to green light (and inherently blue, hence the need for the yellow filter). Associated with Magenta Dye Developer molecules.
- Red-Sensitive Layer: Contains silver halide crystals sensitive primarily to red light (and inherently blue). Associated with Cyan Dye Developer molecules.
- Interlayers: Thin layers separating the emulsions, controlling chemical interactions.
- Black Base Layer: An opaque plastic base providing structure and preventing light leakage from behind.
Capturing Light: The Physics and Photochemistry of the Latent Image
When you press the shutter, the focused light from the lens strikes these light-sensitive layers. Here’s the physics at play:
- Photon Interaction: Light consists of photons, discrete packets of energy. When photons of the appropriate energy (corresponding to blue, green, or red light) strike the silver halide (AgX, typically silver bromide with some iodide) crystals within their respective sensitive layers, they interact with the crystal lattice.
- Photoelectric Effect Principle: A photon can excite an electron within the crystal (specifically, from a bromide ion), freeing it to move. This is analogous to the photoelectric effect, a cornerstone of quantum physics.
- Latent Image Formation (Photochemistry): This mobile electron eventually gets trapped at a sensitivity speck (an imperfection in the crystal). This trapped electron attracts a mobile silver ion (Ag+). The silver ion accepts the electron and is reduced to a tiny, stable speck of metallic silver (Ag). This process repeats, forming invisible clumps of metallic silver where light has struck. This collection of silver specks across all layers constitutes the latent image – an invisible recording of the light pattern.
The Magic Unfolds: Physics of Diffusion and Chemical Development
This is where the process becomes truly fascinating after the rollers spread the reagent:
- Reagent Activation: The highly alkaline reagent (a viscous goo, often containing potassium hydroxide or sodium hydroxide) permeates the layers. It serves multiple roles:
- Activates the developing agents.
- Contains an opacifier (like titanium dioxide) making the developing film initially opaque to shield the sensitive layers from ambient light.
- Provides the medium for chemical reactions and diffusion.
- Selective Development (Redox Chemistry): The developing agents in the reagent are powerful reducing agents. They find the latent image specks (metallic silver).
- Exposed Areas: Where light hit and formed latent image silver, the developing agents rapidly reduce more silver ions in the exposed silver halide crystal to metallic silver. Crucially, during this reduction process, the developing agent itself gets oxidized. This oxidation reaction also affects the nearby Dye Developer molecule associated with that layer, causing it to become oxidized and immobilized.
- Unexposed Areas: Where light didn’t hit, there’s no significant latent image. The developer reacts much slower or not at all with the unexposed silver halide. Consequently, the associated Dye Developer molecule remains unoxidized, soluble, and mobile.
- Dye Diffusion (Physics of Diffusion): This is a critical physics principle. The mobile, unoxidized dye developer molecules (Yellow from unexposed blue-sensitive areas, Magenta from unexposed green-sensitive areas, Cyan from unexposed red-sensitive areas) are now free to move. Driven by a concentration gradient (moving from an area of high concentration in their original layer to low concentration elsewhere), they diffuse upwards through the layers towards the image-receiving layer. The previously mentioned yellow filter layer dissolves in the reagent, allowing underlying dyes to pass through.
- Positive Image Formation (Subtractive Color & Chemistry): As these mobile dyes reach the image-receiving layer, they chemically bond to the mordant molecules within it, becoming trapped and fixed.
- Where the original scene was red, the red-sensitive layer was exposed, immobilizing the cyan dye developer. Blue and green layers were unexposed, allowing yellow and magenta dye developers to migrate to the image layer, combining to form red (Yellow + Magenta = Red in subtractive mixing).
- Where the original scene was green, the green-sensitive layer was exposed (immobilizing magenta), red/blue were unexposed (allowing cyan/yellow to migrate), forming green (Yellow + Cyan = Green).
- Where the original scene was blue, the blue-sensitive layer was exposed (immobilizing yellow), red/green were unexposed (allowing cyan/magenta to migrate), forming blue (Magenta + Cyan = Blue).
- Where the original scene was white (all colors present), all layers were exposed, immobilizing all dyes. No dyes migrate, leaving the receiving layer white.
- Where the original scene was black (no light), no layers were exposed, allowing all three dyes (C, M, Y) to migrate. They combine on the receiving layer to form black (Cyan + Magenta + Yellow = Black). This process creates a full-color positive image using the principles of subtractive color mixing.
Stopping Time: Neutralization and Image Stability
The development process can’t go on forever. The timing and acid layers perform the final crucial step:
- Controlled Timing: The timing layer(s) act as a physical barrier, controlling the rate at which the alkaline reagent diffuses downwards towards the acid layer. This ensures sufficient time for dye development and diffusion. The physics of diffusion through porous media governs this rate.
- Neutralization (Acid-Base Chemistry): Once the alkali penetrates the timing layer(s) and reaches the polymeric acid layer, a rapid acid-base neutralization reaction occurs. This drastically lowers the pH of the chemical environment, deactivating the developing agents and stopping any further dye migration or chemical changes. This stabilizes the final image.
Modern Instant Film Physics (Instax, I-Type)
While the core principles remain remarkably similar, modern instant films like Fujifilm’s Instax and Polaroid’s current I-Type and Go films feature refinements:
- Different Layer Structures: Optimized layer arrangements and thicknesses.
- Improved Chemical Stability: Better dye stability and image permanence (though still sensitive to UV light).
- Development Mechanism: Instax film, for example, completes its development entirely behind a protective opaque layer that is part of the film structure itself, rather than relying solely on an opacifier within the reagent that clears over time. The image appears as if “pushed” to the viewing surface. Peel-apart instant films (now largely discontinued) worked differently, requiring manual separation of the positive print from the negative and chemical sheet.
Conclusion: The Elegant Physics and Chemistry Behind the Polaroid
Understanding how Polaroids work through the lens of physics reveals an extraordinary feat of engineering. It seamlessly integrates principles of optics (light focusing, exposure), quantum physics (photon interaction, photoelectric effect), fluid dynamics and pressure (reagent spreading), thermodynamics and kinetics (diffusion rates, reaction speeds), and materials science (multi-layer thin films), all working in concert with sophisticated photochemistry and redox reactions.
Dr. Edwin Land didn’t just invent a camera; he invented a portable, automated chemical laboratory capable of capturing light and rendering a full-color image in minutes. The enduring magic of watching a Polaroid picture develop is amplified when one appreciates the complex, yet elegant, scientific principles orchestrating that seemingly simple transformation. It remains a powerful demonstration of applied physics and chemistry, creating tangible memories in an increasingly digital world.